Abstract
Introduction: Acute lymphoblastic leukemia (ALL) is the most common acute leukemia in pediatric patients, but accounts for only 20% of acute leukemia diagnoses in adults. Vincristine (VCR) is a cornerstone chemotherapy agent in the management of ALL, but is known to cause high rates of chemotherapy induced peripheral neuropathy (CIPN). Tyrosine kinase inhibitors (TKIs) are routinely incorporated into the treatment of Philadelphia chromosome positive (Ph+) ALL, and have been shown to have potential neuroprotective effects in the prevention of CIPN. Pre-clinical work using animal models has suggested the attenuation of VCR CIPN with the TKI nilotinib. Based on these pre-clinical results, we sought to evaluate whether TKIs had a protective effect on the development of CIPN in patients with ALL treated with VCR containing regimens.
Methods: We performed a retrospective study on adult ALL patients who were treated with VCR-based regimens from 1/1/11 to 12/31/20 at the Ohio State University James Cancer Hospital. Demographics including age, gender, race/ethnicity, relationship status, and drug and alcohol use were collected. Clinical information was also collected including performance status, height/weight, subtype of ALL and date of diagnosis, chemotherapy regimen, comorbidities, falls, date of last follow-up, and information on CIPN. Descriptive statistics were generated on all patients, stratified by those who received a TKI and those who did not. Chi-squared tests were performed to assess for an association between presence and severity of CIPN and TKI use and to evaluate other significant factors associated with the development of CIPN. Given that the majority of TKI patients received hyper-CVAD (cyclophosphamide, VCR, doxorubicin hydrochloride, and dexamethasone), a sub-analysis was performed for all patients treated with hyper-CVAD.
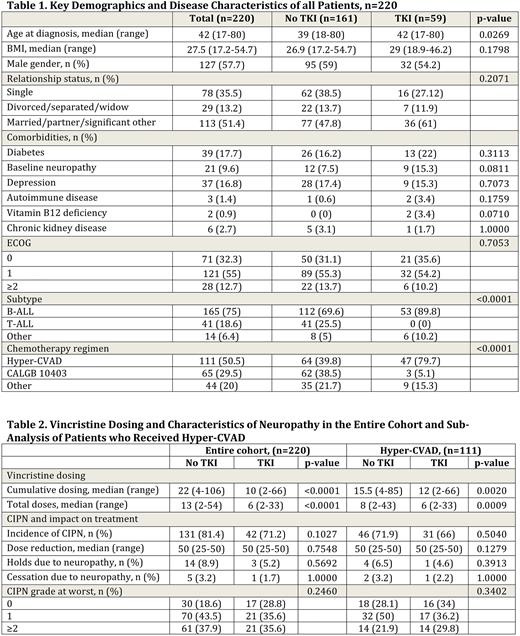
Results: A total of 220 patients were included in the analysis, with a summary of key patient characteristics shown in Table 1. Median age at diagnosis was 42 (range 17-80) and 57.7% of patients were male. The majority of patients had B-cell ALL (75%), with 18.6% T-cell ALL and 6.4% other. The most common treatment regimen was hyper-CVAD (50.5%), followed by the CALGB 10403 (Stock Blood 2019) regimen (29.5%). Approximately one quarter (26.8%) of patients received TKIs, with the large majority (96.6%) receiving dasatinib and 1.7% receiving either nilotinib or ponatinib.
The presence of baseline peripheral neuropathy and lower body mass index (BMI) were the only statistically significant variables between the no CIPN group (grp) and the CIPN grp (P=0.0092, 0.0359). Median BMI was 30 (range 18.9-54.7) in the no CIPN grp as compared to 27.2 (range 17.2-52.9) in the CIPN grp. Cumulative dosing and total doses of VCR was not significant between the no CIPN and CIPN grps (P=0.2675, 0.0630). When comparing those patients who received TKI (TKI grp) to those who did not (no TKI grp), there was a trend toward lower rates of CIPN in the TKI as compared to the no TKI grp at 71.2% compared to 81.4%, but no significant difference was seen (P=0.1027, Table 2). In the sub-analysis of patients who received hyper-CVAD (n=111), 66% of pts in the TKI grp developed CIPN as compared to 71.9% in the no TKI grp, with no significant difference seen (P=0.504, Table 2). For both the entire cohort and hyper-CVAD sub-analysis, there was no significant difference in max dose reduction, holds or cessation due to neuropathy, or grade at worst seen between the grps (Table 2). For both analyses, the no TKI grp also received significantly higher cumulative doses and total doses of VCR (Table 2).
Conclusions: Overall, we found no significant differences in the development of neuropathy in patients with ALL treated with VCR-based regimens with or without TKI, despite higher doses of VCR utilized in patients treated without TKIs. However, the majority of our patients received dasatinib rather than nilotinib, which has been the focus of recent pre-clinical studies. Our findings may suggest a potential mechanistic difference in the effects of dasatanib and nilotinib on the neuronal transport of VCR. While our findings should be confirmed in a larger analysis, they suggest that dasatinib lacks a protective effect against neuropathy for ALL patients treated with VCR-based regimens. Based on promising preclinical data, future studies should focus on the impact of nilotinib for neuroprotection in the prevention of CIPN.
Disclosures
Mims:Servier: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Other: Data Safety and Monitoring Board; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; Genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. McNally:Paradigm Medical Communications, LLC: Consultancy, Honoraria; JADPRO: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal